Since vaccines are administered to otherwise healthy people, they are among the most rigorously tested and safest medical products on the market. In fact, it can take 15 to 20 years – or more – and approximately $1 billion dollars to thoroughly test a new vaccine before it is licensed and made available to the public. The COVID-19 vaccines did not take nearly as long to create, but that does not mean that the vaccines are not safe. Learn more on our COVID-19 FAQ page.
On this page, we address some of the most common questions people have about vaccine safety.
[accordion clicktoclose=true tag=h3]
[accordion-item title=”What are the risks vs. benefits of vaccines?”]
Not vaccinating yourself and your family is not a risk-free decision. Vaccines can prevent infectious diseases that once killed or seriously harmed many children, teens and adults. Without vaccines, your family is at risk for getting seriously sick and suffering pain, disability and even death from diseases like COVID-19, measles, whooping cough and flu.
Even though some of the vaccine-preventable diseases that we protect against are rare in the U.S., they still occur around the world, and unvaccinated travelers can easily bring these diseases with them to the U.S., putting unvaccinated people of all ages at risk of serious illness.
All vaccine-preventable diseases are not the same – some are more deadly than others; and some are more contagious than others. But whether the chance of getting sick or dying from a particular disease is 1 in 100 or 1 in 10,000, you must decide if the risk is worth taking with your health. No one ever thinks that their child will be the 1 in 10,000 that will die from the vaccine-preventable disease.
On the other hand, the risks associated with getting vaccines are side effects that are almost always mild (redness, pain or swelling where the shot was given, or a low-grade fever) and go away within a few days. Serious side effects after vaccination, such as a severe allergic reaction, are extremely rare and healthcare providers are trained to deal with them. In fact, a child is 100 times more likely to get struck by lightning than have a severe allergic reaction to a vaccine.
The disease prevention benefits of getting vaccines are much greater than the possible side effects for almost everyone. The only exceptions to this are people who have specific health conditions, such as a very weak immune system, or if they had a severe allergic reaction to a previous vaccine dose. Learn more about who shouldn’t get certain vaccines or should wait before getting vaccinated.
Please talk to your healthcare provider and consider the proven benefits and risks of each vaccine before making any decisions that can affect the health of your family.
The CDC’s Vaccine Information Statements (VIS), which are created for every vaccine recommended in the United States, explain both the benefits and risks of the vaccines.
[/accordion-item]
[accordion-item title=”How are vaccines tested and monitored for safety?”]
Vaccines are one of the most thoroughly tested medical products available in the U.S. Before a vaccine can be considered for approval – or emergency use authorization – by the FDA, a vaccine manufacturer must show it is safe and effective through clinical trials. Developing a new vaccine begins with exploratory stage and pre-clinical stage before advancing to three stages of clinical trials. Together, this scientific process can take over a decade and cost millions of dollars. The FDA then examines these studies and determines whether a vaccine is safe, effective, and ready to be licensed for use. The FDA only licenses vaccines that have data that shows that the vaccines’ benefits outweigh the potential risks. If there is any question about the data, or any holes in the data, the FDA will request further studies before approving the vaccine.
After the FDA approves a vaccine, the Advisory Committee on Immunization Practices (ACIP) – a group of public health and medical experts – carefully reviews all of the data from the vaccine’s clinical trials and any other studies available to decide whether or not to recommend it and if recommended, for what ages. The CDC Director then reviews the ACIP’s recommendations and decides whether or not to officially approve them. If approved, they become part of the recommended immunization schedules. The ACIP continues to monitor vaccine safety and effectiveness data even after the vaccine’s routine use and may change recommendations based on that data.
After a vaccine is licensed by FDA and recommended for use by the CDC, there are a number of systems in place that work together to help scientists monitor the safety of vaccines and identify any rare side effects that may not have been found in clinical trials. Even large clinical trials may not be big enough to find very rare side effects. For example, some side effects may only happen in 1 in 100,000 or 1 in 500,000 people. Second, vaccine trials may not include certain populations like pregnant women or people with specific medical conditions who might have different types of side effects or who might have a higher risk of side effects than the volunteers who got the vaccine during clinical trials.
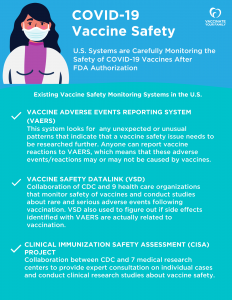
The COVID-19 vaccines – like all vaccines – are being very carefully monitored for safety. In addition to the existing U.S. vaccine monitoring systems listed below, there are also additional systems including CDC’s smartphone-based v-safe. Click on the two graphics below for more information or visit CDC.
VAERS is a reporting system that relies on individuals to report vaccine reactions. Anyone can report a reaction or injury, including healthcare providers, patients and patients’ representatives, such as caregivers or attorneys. The system is co-managed by the FDA and the CDC. However, it is important to note that VAERS data alone can’t be used to answer the question, “Does a certain vaccine cause a certain side effect?” This is because adverse events reported to VAERS may or may not be caused by vaccines. There are reports in VAERS of common conditions that occur just by chance after vaccination. Further investigation may find no medical link between vaccination and these conditions. Instead, the purpose of VAERS is to see if unexpected or unusual patterns emerge, which may indicate a vaccine safety issue that needs to be researched further.
Created in 1990, VSD is a collaboration between the CDC’s Immunization Safety Office and nine health care organizations across the country. VSD monitors the safety of vaccines and conducts studies about rare and serious adverse events following vaccinations.
CISA, which was created in 2001, is a national network of vaccine safety experts from the CDC’s Immunization Safety Office, seven medical research centers and other partners. CISA addresses vaccine safety issues, conducts high quality clinical research and assesses complex clinical adverse events following vaccination. CISA also helps to connect clinicians with experts who can help consult on vaccine safety questions related to individual patients.
PRISM – part of FDA’s Sentinel Initiative – is a partnership between the FDA’s Center for Biologics Evaluation and Research (CBER) and leading health insurance companies. It actively monitors and analyzes data from a representative subset of the general population. PRISM links data from health plans with data from state and city immunization information systems (IIS). PRISM has access to information for over 190 million people allowing it to identify and analyze rare health outcomes that would otherwise be difficult to assess. See vaccine studies done using PRISM data.
To view how the COVID-19 vaccine is being tested and monitored for safety, visit COVID-19 FAQ. You can also download the CDC’s The Journey of Your Child’s Vaccine infographic.
What about the Safety of COVID-19 Vaccines?
While COVID-19 vaccines were developed faster than other vaccines, safety is a top priority. According to CDC, “these vaccines have undergone and will continue to undergo the most intensive safety monitoring in U.S. history. “
Before COVID vaccines are authorized/approved for use by FDA:
- Each COVID-19 vaccine was tested through three phases of clinical trials.
- After the clinical trials, the data was carefully reviewed by the FDA and their advisory committee (VRBPAC), and the CDC and their advisory committee (ACIP)
Since they were authorized/approved for use by the FDA:
- Millions of people in the U.S. have received COVID-19 vaccines.
- COVID vaccines continue to be monitored for safety using both established systems like VAERS and new safety monitoring systems like V-Safe.
Learn more about COVID-19 vaccines and how they monitored for safety on our COVID Vaccine Q&A page.
You can also view and download CDC’s vaccine safety handout.
Data shows that getting a COVID-19 vaccination is a safer and more dependable way to build immunity to COVID-19 than getting sick with COVID-19.
[/accordion-item]
[accordion-item title=”How do you know that it is safe to give my child different vaccines at the same time/during the same office visit?”]
Before every new vaccine is licensed by the FDA, it is tested along with the vaccines already recommended for children at that particular age. (These are called concomitant use studies). The new vaccines must prove they are safe and effective when given in combination with already approved vaccines that will be given at the same time. Additionally, scientific studies have confirmed that getting several vaccines at the same time does not cause any chronic health problems (see our resource section for the studies). Sometimes, certain combinations of vaccines given together can cause fever, and occasionally febrile seizures occur as a result of this fever. However, febrile seizures are temporary and do not cause any lasting damage. In fact, febrile seizures can also occur when a child has a fever from an illness. Based on this information, both the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) recommend getting all childhood vaccines according to the CDC’s recommended immunization schedule.
A combination vaccine is two or more different vaccines that have been combined into a single shot. With combination vaccines, people get the same protection as they do from individual vaccines given separately, but they receive fewer shots. As with individual vaccines, before a combination vaccine is approved and recommended for use in the U.S., it goes through rigorous testing to make sure it is safe and effective with the rest of the recommended immunization schedule. And just like for individual vaccines, safety systems are in place after the combination vaccines are recommended to the public to watch for even very rare side effects.
Giving several shots at the same time means fewer office visits for the millions of babies born each year and it also reduces the chance that the children will miss getting their recommended vaccinations on time. Getting combination vaccines has been shown to cause less stress on babies.
Read the blog post Debunking myths about vaccine testing and safety by
[/accordion-item]
[accordion-item title=”Are combination vaccines safe?”]
Yes, combination vaccines are safe. A combination vaccine is two or more different vaccines that have been combined into a single shot. With combination vaccines, people get the same protection as they do from individual vaccines given separately, but they receive fewer shots. Sometimes combination vaccines cause slightly more pain or swelling where the shot was given. But if you (or your child) got the shots individually, you might have pain or swelling in two or three spots, instead of just one.
Examples of combination vaccines for children in the U.S. include:
- Pediarix – combines DTaP, Hepatitis B, and IPV (polio)
- ProQuad – combines MMR and varicella (chickenpox)
- Kinrix – combines DTaP and IPV
- Pentacel – combines DTaP, IPV, and Hib
The MMR vaccine (measles, mumps, and rubella), the DTaP vaccine (diphtheria, tetanus, and pertussis) for young children and the Tdap vaccine (tetanus, diphtheria and pertussis) for adolescents and adults each protect against three diseases, but aren’t called combination vaccines in the U.S. This is because you cannot get separate vaccines for all of the diseases that MMR, DTaP, and Tdap protect against.
As with individual vaccines, before a combination vaccine is approved and recommended for use in the U.S., it goes through rigorous testing to make sure it is safe and effective when given with the rest of the recommended immunization schedule. And just like for individual vaccines, safety systems are in place after the combination vaccines are recommended to the public to watch for even very rare side effects.
Getting combination vaccines has been shown to cause less stress on babies.
[/accordion-item]
[accordion-item title=”How is the amount in each vaccine dose decided? How can a baby get the same dose of a vaccine as an older child or adult?”]
Vaccine doses are not randomly chosen. During the development of a vaccine, different doses are tested to determine the lowest amount that will be effective for those who will be getting the vaccine.
Although people aren’t the same, their immune system response is similar. For most vaccines, the same vaccine dose can be given to people of different ages, but there are exceptions. In some cases, children and adults need different amounts of the same vaccine because our immune system weakens with age. For example, the dose of hepatitis B vaccine for adults is higher than that the dose used in infants. In other cases, different versions of vaccines are available for different age groups, such as DTaP vaccine (diphtheria, tetanus and pertussis) for young children, and Tdap vaccine (tetanus, diphtheria, and pertussis) for older children, teens, adults and pregnant women. Or, the number of doses needed may be different depending on the age of the person. For example, some children between 6 months and 8 years old will require two doses of flu vaccine in order to get adequate protection from flu.*
*Sources: AAP’s Healthy Children and CHOP’s Vaccine Education Center.
[/accordion-item]
[accordion-item title=”Who creates the immunization schedules in the U.S.?”]
The Centers for Disease Control and Prevention (CDC) sets the U.S. immunization schedules based on recommendations from the Advisory Committee on Immunization Practices (ACIP). The ACIP is a group of medical and public health experts who carefully review all available data about each vaccine from clinical trials and other studies to develop vaccination recommendations for children, adolescents and adults (including pregnant women). Before making any recommendations, the ACIP conducts extensive research on the safety and effectiveness of each vaccine given alone, as well as when given in combination with other vaccines on the schedule.
ACIP’s recommendations include the age(s) when the vaccine should be given, the number of doses needed, the amount of time between doses, and precautions and contraindications. The ACIP meets three times a year to make recommendations for new vaccines and to make changes, if needed, to recommended vaccinations based on the latest science and research. Meetings are open to the public and available online via webcast. The recommendation schedules for all ages are reviewed and updated, if needed, every year.
The CDC’s recommended childhood immunization schedule (from birth to 18 years old) is the ONLY vaccination schedule for children and teens that is rigorously tested for safety and effectiveness. Therefore, it is very important that parents follow that schedule. The vaccines are carefully timed to provide protection to your children when they are most vulnerable to diseases, and when the vaccines will produce the strongest response from your children’s immune systems.
No “non-standard” or “alternative” childhood vaccination schedules have ever been tested for their safety and effectiveness, and therefore, it can be dangerous for children to follow them. So, although following a non-standard schedule or creating your own vaccine schedule may seem like a good idea, please speak to your child’s healthcare provider and make sure you are making these decisions based on complete, science-based information.
Download the CDC’s ACIP fact sheet.
Learn more about the vaccine schedule from our experts.
[/accordion-item]
[accordion-item title=”Is it dangerous for my child to get so many vaccines when he is so young?”]
No, it is not dangerous. The vaccines in the CDC’s recommended childhood immunization schedule are carefully timed to provide protection to your child when he or she is most vulnerable to diseases, and when the vaccines will produce the strongest response from your child’s immune system. While it’s understandable that you may be concerned that your child is too young for the recommended vaccinations or you may feel that there are too many vaccines given to your child at once, it is important to understand that young children can easily be exposed to a disease and therefore they are never too young to be protected with a vaccine.
[/accordion-item]
[accordion-item title=”If I’m breastfeeding my baby, why do I need to vaccinate him or her?”]
Babies’ immune systems are not fully developed at birth, which puts newborns at greater risk for infections. Even though your baby may get some short-term immunity from you during the last few weeks of pregnancy, it is only for diseases to which you are immune from or vaccinated against.
Even breastfed babies need to be protected with vaccines at the recommended ages. While breast milk provides important, temporary protection from some minor infections like colds, ear infections and diarrhea, as your baby’s immune system is developing, breast milk will not protect him or her against all diseases. Your baby needs the long-term protection that can only come from making sure he or she receives all his vaccines according to the recommended immunization schedule, before she is exposed to diseases.
The CDC’s recommended childhood immunization schedule (from birth to 18 years old) – which is also recommended by the American Academy of Pediatrics and the American Academy of Family Physicians – is the ONLY vaccination schedule for children and teens that is rigorously tested for safety and effectiveness. Therefore, it is very important that parents follow that schedule. No “non-standard” childhood vaccination schedules have ever been tested for their safety and effectiveness, and therefore, it can be dangerous for children to follow them. So, although following a non-standard schedule or creating your own vaccine schedule may seem like a good idea, please talk to your child’s doctor and make sure you are making these decisions based on science-based information.
[/accordion-item]
[accordion-item title=”How can I be confident that vaccines don’t cause autism?”]
Vaccines do not cause autism. Nearly a dozen studies and reviews following millions of children conducted worldwide over the last decade have failed to find a connection between autism and childhood vaccines. In fact, the original, singular study that claimed to find a connection has been officially retracted by the publication when it was found the lead author, Dr. Andrew Wakefield, lied about his findings. He has since lost his license to practice medicine.
Both the medical and public health communities that monitor vaccine safety have heard the claims of a vaccine-autism link, researched them extensively, and repeatedly disproved them. We encourage you to look at the evidence yourself by reading the many studies that show no link between vaccinations and autism.
Parents can be confident that the medical and public health communities strongly support the safety and benefits of immunizations. These communities include the prestigious National Academy of Medicine (NAM) (formerly known as the Institute of Medicine or IOM), the American Academy of Pediatrics (AAP), the American Academy of Family Physicians (AAFP), the American Medical Association (AMA), the World Health Organization (WHO), the National Institutes of Health (NIH), the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and MANY others.
Learn more about this issue and the promising research on autism on the Autism Science Foundation’s website.
Alison Singer is the mother of a daughter with autism. Watch this video below as she describes her own personal journey to becoming an autism advocate and investigating the alleged connection between autism and the MMR vaccine.
View more about the Autism-Vaccine Myth from PBS NOVA.
[/accordion-item]
[accordion-item title=”Where can I find the studies done on the safety of vaccines?”]
Extensive research has been conducted to determine the ongoing safety and effectiveness of vaccines for people of all ages. Vaccinate Your Family has collected these studies so that the public has easy access. Learn more about the science behind the safety and efficacy of vaccines by visiting our Vaccine Research section.
[/accordion-item]
[accordion-item title=”Are vaccines safe?”]
Yes, our organization is confident in the safety of vaccines because of our deep understanding of the complex systems in place to license safe vaccines and continually monitor their safety after being licensed. As vaccines are given to otherwise healthy people, they are held to the highest safety standards, requiring more rigorous testing than most medications. It can take 15 or more years and an average of $1 billion dollars to thoroughly test a new vaccine before it is licensed by the Food & Drug Administration (FDA) and made available to the public.
To help understand the process of developing, licensing and monitoring vaccines, read the answer to the question on this page – How are vaccines tested and monitored for safety? Additionally, the CDC created a very helpful infographic: The Journey of Your Child’s Vaccine.
As with any medication, side effects can occur after vaccination. However, these side effects are usually minor and most often include soreness or redness at the site where the shot was given, and a low fever (which is actually a positive sign that the body is doing its job by reacting to the vaccine). Severe reactions to vaccines are very rare. Information about possible adverse events is available in the ACIP’s recommendations for each vaccine. Information for the public on possible side effects after vaccination can be found on each vaccine’s Vaccine Information Statement (VIS).
[/accordion-item]
[accordion-item title=”Isn’t natural immunity better than vaccines?”]
Natural immunity occurs when a person is exposed to a disease (virus, bacteria, germ), becomes infected and survives the battle against the disease. While it is true that natural immunity usually results in better immunity than vaccination, the risks you are taking with your family’s health are much greater. You never know how a disease will affect you or someone in your family. For instance, a natural chickenpox infection may result in pneumonia or another serious complication leading to hospitalization or even death, whereas the chickenpox vaccine might only cause a sore arm for a couple of days. Measles, meningitis, polio and many other vaccine-preventable diseases can kill its victims or leave them seriously debilitated for life.
When it comes to the COVID-19 vaccines, studies have shown that getting a COVID-19 vaccination is a safer and more dependable way to build immunity to COVID-19 than getting sick with COVID-19.
Listen to a brief video by VYF Board Member Dr. Paul Offit as he explains why natural immunity comes at a serious price.
According to the CDC, the immune system is not fully developed at birth, which puts newborns at greater risk for infections. Even though a baby may get some short-term immunity from the mother during the last few weeks of pregnancy, it is only for diseases to which the mother is immune to or vaccinated against. Even breastfed babies need to be protected with vaccines at the recommended ages.
While breast milk provides important protection from some infections (such as colds, ear infections and diarrhea) as your baby’s immune system is developing, breast milk does not protect children against all diseases. Your baby needs the long-term protection that can only come from making sure he receives all his vaccines according to the CDC’s recommended immunization schedule, before he is exposed to diseases.
[/accordion-item]
[accordion-item title=”Why don’t we do a study of vaccinated vs. unvaccinated children?”]
Since vaccines are administered to otherwise healthy people, they are among the most rigorously tested and safest medical products on the market. It can take 15 to 20 years – or more – and approximately $1 billion dollars to thoroughly test a new vaccine before it is licensed and made available to the public. There have been numerous research studies done on the efficacy and safety of vaccines. Visit our Vaccine Research section to view large number of studies yourself. You can also see a chart of vaccination studies on AAP’s website.
The reason we don’t do studies to compare large groups of vaccinated vs. unvaccinated children is a very simple reason of medical ethics. When vaccines exist and are available to prevent against diseases, it would be unethical for scientists to assign children to a study’s “control group” where they don’t get vaccinated and are thereby unprotected from vaccine-preventable diseases. Especially, when vaccinating is considered a “standard-of-care”. There is no place in the world where an ethical committee would approve of scientists asking parents not to vaccinate their children, even for the purpose of a study.
While it is not ethical to do large, double-blinded, randomized controlled trials comparing our current vaccine schedule (vaccinated) to placebo (unvaccinated), there have been a few studies that have looked at the health status of vaccinated vs. unvaccinated children and adolescents. For example, Vaccination Status and Health in Children and Adolescents: Findings of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS), which showed “the prevalence of allergic diseases and non-specific infections in children and adolescents was not found to depend on vaccination status.”
Read more about what would be required to conduct a scientifically-sound study of unvaccinated vs. vaccinated children from pediatrician Dr. Chad Hayes.
[/accordion-item]
[accordion-item title=”Aren’t vaccines just a way for pharmaceutical companies (Big Pharma) to make money?”]
While vaccine manufacturers, like manufacturers of many other products sold in the U.S. do make money from vaccines, vaccines don’t make them as much money as their other medicines like Humira, Keytruda, Eliquis and others. Vaccines are not even in the top 5 of money-making pharmaceutical products.
Vaccines are actually considered “cost-saving”. That means that by vaccinating people of all ages, we are saving money. For example, for every $1 spent on childhood vaccinations, the U.S. saves $10.10. And for the children vaccinated each year, the country ends up saving $13.6 billion in direct medical costs by preventing infectious diseases in the first place. Additionally, outbreak responses also take a lot of time, money and manpower. Through timely vaccinations, the U.S. saves billions of dollars. Learn more about the costs of outbreak response and treating illness among unvaccinated people.
Most importantly, vaccines are lifesaving. Without vaccines, all of the preventable diseases that hospitalized, disabled and killed countless numbers of people in the U.S. and around the world will come back causing unimaginable loss. For example, polio, which caused approximately 50,000 cases each year in the U.S., was one of the most dreaded childhood diseases of the 20th century with annual epidemics. But, through successful vaccination programs around the world, polio is almost gone. Read some of the stories from people affected by vaccine-preventable diseases.
[/accordion-item]
[accordion-item title=”What does the Jewish religion say about vaccinating?”]
In 2019, the United States had large measles outbreaks with 75% of the cases in New York. The NY outbreaks – in Rockland County, Brooklyn and Queens – began after Orthodox Jews who were visiting Israel became infected with measles and brought the disease with them to the U.S. They then spread it to Orthodox communities with pockets of unvaccinated people. (Israel is experiencing a massive outbreak of the disease, with about 4,300 cases reported between July 2018 and July 2019.)
Following are statements (and related links) from the Jewish community about the importance of vaccinations.
- The Orthodox Union (OU) and the Rabbinical Council of America (RCA) strongly urge all parents to vaccinate their healthy children on the timetable recommended by their pediatrician. Read their full statement.
- Open Letter from Rav Moshe Sternbuch, Chief Rabbi of the Orthodox Rabbinical Court in Jerusalem, to Rav Malkiel Kotler About The Halachic Requirement To Vaccinate. In his letter Rav Sternbuch states “Since it is proven that vaccines are effective to prevent the spread of disease, it is an obligation upon every father to vaccinate his children.” Read the full letter on The Yeshiva World website.
- Rav Asher Weiss ,chief halachic authority for Shaarei Tzedek Medical Center in Yerushalayim, stated “Vaccinating children against preventable diseases has the same halachic necessity for parents as taking them to the doctor for an illness” at a conference on medical ethics in Boro Park. Read more about Rav Weiss’ comments about vaccinations during the conference.
- Orthodox Jewish Nurses Association (OJNA) Vaccine Task Force – The Task Force is headed by Blima Marcus, an ultra-Orthodox oncology nurse at the Memorial Sloan Kettering Cancer Center and an adjunct professor of nursing in Hunter college from Borough Park, Brooklyn. Listen to Marcus discuss the OJNA Vaccine Task Force.
- A 2005 ruling by the Conservative movement’s Rabbinical Assembly allows Jewish day schools to make immunizations compulsory in accordance with halacha (Jewish law). Read the full ruling: Compulsory Immunization in Jewish Day Schools.
- Central Hatzolah has joined with the NYC Health Department and Released a Letter Calling on the Community to Vaccinate.
- The Union for Reform Judaism’s (URJ) Resolution on Mandatory Immunization Laws states “Throughout the history of the Jewish people, one of the most sacred commandments has been pikuach nefesh, the idea that the preservation of life takes precedence over almost all other Jewish laws.” They also state “A 1999 CCAR responsum on Compulsory Immunization (5759.10), finds that vaccines qualify as refu’ah bedukah, proven remedies, and are therefore an obligation.” The URJ affirms that in the case of mandatory immunizations, pikuach nefesh and refu’ah bedukah are our guiding principles. Reform Jewish religious tenets prioritize protecting the health of all individuals, the most medically vulnerable members of the community, and the community as a whole, and do not provide a basis for a religious exemption from mandatory immunizations. Read the full resolution.
- Are Vaccinations Kosher? – In Judaism, no religious law forbids vaccination, even in cases where the vaccine includes gelatin and/or other non-kosher ingredients. According to Rabbi David Samson, “There is no prohibition in using medicines which contain forbidden ingredients if they are administered by injection, suppository, enema, medicated bandage, and the like, since they are not eaten.” Read the full answer to this question.
Related Articles
NY Times – Religious Objections to the Measles Vaccine? Get the Shots, Faith Leaders Say
NY Times Opinion – My Fellow Hasidic Jews Are Making a Terrible Mistake About Vaccinations: We should stop dismissing scientific evidence and endangering the lives of our children.– By Moshe Friedman, a Hasidic yeshiva graduate and a father of three.
Kveller – These Amazing Orthodox Nurses Are Fighting Measles in Their Community
Judiasm and Science – A Nice Jewish Shot: Why Vaccinations are Kosher and Required
The Daily Beast – I Didn’t Vaccinate My Child—And I Regret It: How I finally discovered that vaccinating your kids is the right thing to do – By Sally Kohn, a Jewish mother.
[/accordion-item]
[accordion-item title=”What are the Omnibus Autism Proceedings?”]
The National Vaccine Injury Compensation Program (also referred to as “the vaccine court”), housed in the U.S. Court of Federal Claims, initiated the Omnibus Autism Proceedings (OAP) in July 2002 to consolidate the thousands of claims filed by petitioners claiming that vaccines caused autism. The petitioners’ lawyers formed a Petitioners’ Steering Committee (PSC) in order to consolidate the processes and, in conjunction with the Court, decided that all the cases filed fell within three theories:
- Theory 1: Thimerosal-containing vaccines in combination with the measles-mumps-rubella (MMR) vaccine cause autism.
- Theory 2: Thimerosal-containing vaccines cause autism.
- Theory 3: The MMR vaccine causes autism.
Only the first and second theories were heard. The petitioners’ attorneys withdrew their third theory telling the court that they did not have evidence that was different enough from the first theory to justify another set of three test cases.
Theory 1: Thimerosal-containing vaccines in combination with the MMR vaccine cause autism:
- Cedillo Decision – Special Master Hastings
- Hazlehurst Decision – Special Master Campbell-Smith
- Snyder Decision– Special Master Vowell
- Overall Decision – The Special Masters ruled that thimerosal-containing vaccines, in combination with MMR vaccine, do not cause or contribute to autism.
Theory 2: Thimerosal-containing vaccines cause autism:
- Snyder Decision– Special Master Sweeney
- Dwyer Decision– Special Master Vowell
- King Decision– Special Master Hastings
- Overall Decision – The Special Masters ruled thimerosal-containing vaccines do not cause or contribute to autism.
Additional Resources
- Docket of Omnibus Autism Proceeding – The United States Court of Federal Claims created a docket containing all of the materials related to the Omnibus Autism Proceeding. It was last updated in January 2011.
- The National Vaccine Injury Compensation Program (NVICP) – The NVICP is a no-fault alternative to the traditional legal system for resolving vaccine injury petitions. Sometimes referred to as “the vaccine court”, the NVICP began accepting petitions (also called claims) in 1988. Learn more.
- Dr. Zimmerman’s Testimony During Theory 1 of the Omnibus Autism Proceedings
[/accordion-item]
[/accordion]