Questioning Whether To Get Your Child the HPV Vaccine? Read This
 In June 2006, the first human papillomavirus (HPV) vaccine was licensed for use in the U.S. Rather than celebrate the development of a vaccine to prevent a deadly form of cancer, many parents have instead been misguided by fear. As a result of persistent internet stories and inaccurate myths that question the safety of HPV vaccines, parents continue to refuse or delay HPV vaccines for their children, and one of the most effective ways to prevent cancer is being grossly underutilized.
In June 2006, the first human papillomavirus (HPV) vaccine was licensed for use in the U.S. Rather than celebrate the development of a vaccine to prevent a deadly form of cancer, many parents have instead been misguided by fear. As a result of persistent internet stories and inaccurate myths that question the safety of HPV vaccines, parents continue to refuse or delay HPV vaccines for their children, and one of the most effective ways to prevent cancer is being grossly underutilized.
Although millions of doses of HPV vaccines have been administered in the past 10 years, some parents still fear what may happen if their child gets an HPV vaccine.
What they should fear is what may happen if they don’t.
I offer the following information about HPV because everyone should understand where their fears ought to be directed: at the disease, not the vaccine designed to prevent it.
1) It’s not about sex, it’s about cancer.
Regardless of what parents choose to teach (or not teach) their kids about sex, abstinence or contraception, the HPV vaccine is vital to the health of our children because it protects them from cancer.
By preventing people from contracting certain strains of a highly prevalent infection, we can then prevent the possibility of HPV infections turning into cancerous cells. An HPV infection is often contracted shortly after sexual debut, and can eventually lead to cancers of the cervix, vulva, vagina, penis, anus or throat. Since the majority of these cancers have no formal screening measures, they often go undetected until they are well advanced.
2) Nearly all sexually-active individuals will contract HPV at some point in their lives.
HPV is the most common sexually transmitted infection in the United States and is often referred to as the common cold of the genitals. HPV is not a new virus, but many people are unfamiliar with how dangerous and prevalent it is. Consider these staggering statistics:
- Approximately 80 million Americans (about 1 in 4) are currently infected.
- About 14 million people become newly infected each year.
- About half (49%) of these new infections will be among people ages 15-24.
Not only is HPV infection common, but most people rarely know they’re infected because it typically occurs without any symptoms. Since it’s possible to develop symptoms years after first being infected, it’s especially difficult to diagnose exactly when a person first became infected.
In about 90% of cases, an HPV infection will eventually clear in about a year or two. However, during that time, those infected with HPV are often unknowingly spreading the infection to others.
3) As many as 10% of those infected will eventually develop cancer.
While 90% of people may clear the infection, the other 10% end up developing cancerous cells years, or even decades, after initial exposure. Since there is no way to determine which cases will clear and which will lead to cancer, universal vaccination is the most effective means of prevention.
The following data reveals just how many cancer cases are linked to HPV each year:
Cervical cancer: Almost all cervical cancer cases are caused by HPV and more than 11,000 women in the U.S. alone get cervical cancer each year. When looking at the bigger picture, 528,000 new cases of cervical cancer were diagnosed worldwide in 2012.
Anal cancer: About 91% of anal cancers are caused by HPV and there are approximately 4,300 anal cancers diagnosed each year.
Oropharyngeal cancers: (cancers of the head, neck, throat, mouth, tongue, and tonsils) About 72% are caused by HPV and an estimated 8,400 of these cancers are diagnosed each year.
Vaginal cancer: HPV causes about 75% of vaginal cancers and there are about 500 vaginal cancers diagnosed each year.
Vulvar Cancer: HPV causes about 50% of vulvar cancers and an estimated 2,100 vulvar cancers are diagnosed each year.
Penile Cancer: About 63% of penile cancers are linked to HPV and there are about 600 penile cancers diagnosed each year.
Genital Warts: There are more than 40 types of HPV that specifically affect the genital area. However, 90% of genital warts are caused by HPV types 6 or 11 and about 360,000 people in the U.S. get genital warts each year.
Since there is no test to check one’s overall HPV status, and no standard screening to detect HPV in the mouth or throat, getting an HPV vaccine is an effective way to prevent illness rather than leave people vulnerable to infections that can lead to cancer.
Some argue that since there is a test to screen for cervical cancer that this eliminates the need for vaccination among women. While cervical cancer screenings are vitally important, they don’t prevent infection. Instead, they help identify precancerous lesions. Once lesions are discovered, women may then need to endure various invasive and painful procedures. These may include cone biopsies used to help diagnose precancerous or cancerous cells, and a loop electrosurgical excision procedure (LEEP) often used to burn off precancerous lesions. Additionally, cervical cancer screenings don’t help identify other HPV related cancers or help screen of men or adolescents for HPV. With the vaccine we can prevent cancers before they exist.
4) Surprise…you don’t have to have sex to get HPV.
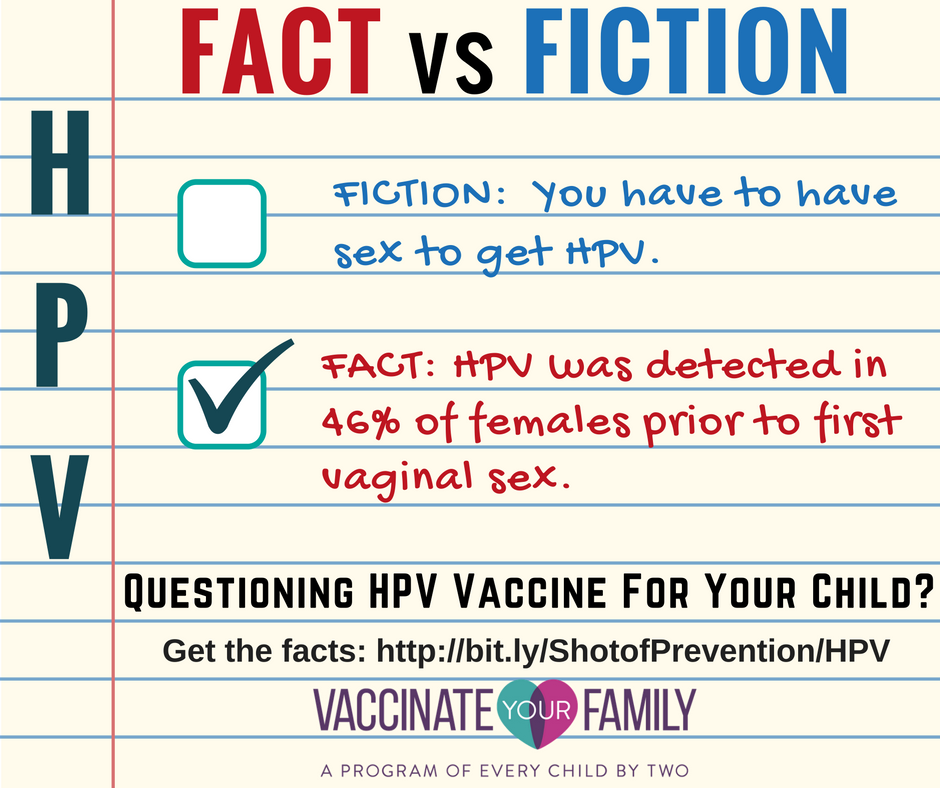
Most people are surprised to learn that HPV transmission is not just limited to vaginal or anal sex. HPV can also be transmitted through intimate skin-to-skin contact. There have even been reports of oral-genital and hand-genital transmission. In fact, one study shows that HPV was detected in 46% of females prior to first vaginal sex.
Based on this information, it’s possible that a person can become infected during their first sexual encounter, even if that encounter doesn’t involve vaginal sex. This is why abstinence until marriage isn’t necessarily effective in preventing HPV infection. Even having one lifetime sex partner doesn’t necessarily eliminate the risk. There’s no guarantee a partner, even if it’s your first or only partner, hasn’t already been infected. In one study, the HPV prevalence among women aged 18–25 years who had only one lifetime sex partner was 14.3%.
While condoms might lower the risk for infection, HPV can infect areas not covered by the condom and therefore even condoms are not a reliable way to fully protect against HPV.
5) HPV vaccines prevent the strains that cause the majority of HPV related cancers.
Currently there are three different HPV vaccines that can be used for routine vaccination and they cover the HPV strains that cause the majority of HPV related cancers.
1.) The first FDA approved HPV vaccine, was a bivalent vaccine known as Cervarix. It is approved for use in females only and offers protection from HPV types 16 and 18 which account for approximately 70% of all cervical cancers worldwide.
2.) The quadrivalent vaccine Gardasil expanded protection to males as well as females, and includes protection against HPV types 16 and 18, but also adds protection against HPV types 6 and 11, which are responsible for approximately 90% of genital warts.
3.) The most recent HPV vaccine to come to market is the 9-valent HPV vaccine known as Gardasil 9. It was licensed by the FDA in December, 2014 and was recommended by the Advisory Committee on Immunization Practices (ACIP) in February, 2015. It is currently licensed for males and females ages 9-26. In addition to targeting HPV types 16 and 18 (like the bivalent) and 6 and 11 (like the quadrivalent), The 9-valent vaccine targets adds five additional cancer causing types (HPV 31, 33, 45, 52, 58).
While the ACIP does not currently suggest a preference between vaccines, researchers have found that the 9-valent vaccine represents a 13% increase in protection compared to to other HPV vaccines. Overall, the 9-valent HPV vaccine increased the protection against cervical cancer to 80% and increased protection against other HPV-related cancers including cancer of the vulva (from 71% to 92%), vagina (73% to 98%), penis (76% to 90%), and anus (87% to 96%). It may also offer protection against an additional 8% of oropharyngeal cancers, including cancer of the base of the tongue and tonsils (the second most common HPV-associated cancer).
The CDC reports that clinical trials of HPV vaccine among persons not previously exposed to a targeted HPV type have shown near 100% vaccine efficacy in preventing cervical pre-cancers, vulvar and vaginal pre-cancers, and genital warts in women caused by the vaccine types, as well as 90% vaccine efficacy in preventing genital warts and 75% vaccine efficacy in preventing anal pre-cancers in men.
It is estimated that if everyone received the 9-valent HPV vaccine as recommended, about 29,000 cancers could be prevented each year.
6) There are several reasons HPV vaccines are recommended beginning at age 11-12.
The HPV vaccine is most effective when the complete series is administered at a young age. There are several reasons for this.
First, it’s best to get vaccinated before exposure, so the best time would be before any sexual activity begins. Statistics show that some children, even as young as 12, have already had sexual encounters that can put them at risk of HPV infection.
Here is the data that we have to go on:
A recent study showed the HPV vaccine is 83% effective in preventing the spread of HPV in women who had never come in contact with the virus but only 53% effective for those who had previous contact with it.
Secondly, studies indicate that the vaccine produces a greater immune response with a higher antibody count to fight infection when given at a younger age.
While some parents have expressed concern that vaccinating a child may make them believe they have permission to have sex, the research indicates that HPV vaccination has had no notable difference in the markers of sexual activity. In other words, the vaccine does not appear to be changing sexual behaviors, only protecting those who will eventually engage in them. To date there has been no notable differences in pregnancies, counseling on contraceptive, and testing and diagnosis of other sexually transmitted infections.
Also, since Tdap and meningococcal vaccine boosters are both recommended at this age, and often required to attend school in most states, providers can use these vaccine appointments to get preteens started on their three dose HPV series.
While there is a recommended dosing schedule which outlines the time between the first and subsequent doses of HPV vaccine, it’s important that parents realize that these are minimum spacing requirements. Subsequent doses can be given at greater intervals if necessary without the need to start the entire dosing schedule over again.
Parents should not delay vaccinating because they are worried that the immunity will wane before exposure. The most current study shows that the vaccine is expected to remain effective for at least eight years after the last dose. However, experts agree there is reason to believe that the protection will extend beyond that. Studies of long-term efficacy are ongoing and will certainly alert us if the immunity should wane and a booster ought to be recommended.
7) Parents should request HPV vaccine even if their provider neglects to mention it.
A recent study was conducted to investigate physician communication about HPV vaccine in which many providers admitted to recommending HPV vaccine inconsistently, behind schedule, or without urgency. There appears to be a number of explanations, none of which are tied to any safety concerns. Some say that they fail to mention it because they’re focused on school vaccination requirements and HPV is currently only required in two states. Some studies suggest doctors are only offering it to the children they believe to be most at risk.
Despite the potential to save lives, HPV vaccination rates have been lower than that for other recommended vaccines. According to CDC data, only 60% of girls ages 13-17 received at least one of the three recommended doses of the HPV vaccine, and only 39.7% had completed the series. Vaccination rates for boys were even lower with 41.7% having received at least one dose and 21.6% percent receiving all three. When compared to the first dose of other vaccines typically received during this age, 79.3% had received a meningococcal vaccines and 87.6% had received the Tdap booster.
While it’s probable that a lack of provider suggestion may be contributing to the low rates of HPV vaccination, a lack of recommendation should not keep parents from seeking the protection their kids deserve and parents should request the vaccination series begin at the recommended age (11-12 years).
8) Sensational stories will continue to circulate, but the science is clear. HPV vaccines are safe.
Inaccurate information about the safety of HPV vaccines is continuously circulating (and recirculating) on the internet. However, to be informed and well-educated on the subject, one must learn that “research” involves more than just believing everything you read on the internet. It involves checking sources and understanding the scientific evidence.
HPV vaccines are not new. From June 2006 through March 2013 approximately 57 million doses of HPV vaccines have been administered and we have an enormous amount of research that demonstrates that the HPV vaccine is not only well-tested, but extremely safe.
The first step in evaluating the safety of any vaccine is to understand the process of vaccine licensing and follow-on safety surveillance.
Prior to a vaccine being licensed there is an extensive period of clinical trials. More than 15,000 participants were included in the clinical trials for the nine-valent vaccine, more than 29,000 participants for the quadrivalent vaccine and more than 30,000 participants for the bivalent vaccine.
Before the nine-valent HPV was licensed by the FDA, the vaccine’s safety was evaluated across seven studies. The safety findings from these pre-licensure studies show it has a similar safety profile to previously approved HPV vaccines.
After vaccines are licensed, the CDC and FDA use three primary systems to continue to monitor and evaluate the safety of vaccines. Any problems detected are then reported to health officials, health care providers, and the public. The three systems are:
- The Vaccine Adverse Event Reporting System (VAERS) – an early warning system that allows anyone to report adverse health events following vaccination to help detect possible new, unexpected, or increased trends in reported adverse events.
- The Vaccine Safety Datalink (VSD) – a collaboration between CDC and several large health care organizations that allows ongoing monitoring and proactive searches of vaccine-related safety data.
- The Clinical Immunization Safety Assessment (CISA) Project – a partnership between CDC and several medical centers that conduct clinical research on vaccine-associated health risks in certain groups of people to help understand how adverse events might be caused by vaccines.
Some of the studies demonstrating the safety of HPV vaccine include the following:
- In a study published August 2009 in the Journal of the American Medical Association (JAMA), VAERS data from June 2006 through December 2008 was thoroughly analyzed. At the time, more than 23 million doses of HPV vaccine had been administered with 12,424 reports of adverse events. Of those reports, 94% of them were deemed “not serious” and 6% were described as “serious”, with the most frequently reported symptoms being headache, nausea, vomiting, fatigue, dizziness, syncope/fainting, and generalized weakness. This is important to mention because personal stories that circulate on the internet often suggest serious events that are not validated.
This study also investigated 32 reports of death. Medical investigations into these cases revealed that there was no common pattern that might suggest these deaths were caused by the vaccine. In cases where there was an autopsy, death certificate, or medical records, the causes of death were explained by factors other than the vaccine. Some causes of death included diabetes, viral illness, illicit drug use, and serious heart conditions. While the deaths may have occurred after vaccination, it is important to understand that the medical investigations proved that they were not a result of vaccination – a point that vaccine critics fail to clarify in their discussions about HPV vaccine safety.
In conclusion, the study found that the safety of the HPV vaccine was consistent with the safety of other vaccines recommended for this age group.
- A 2011 study of VSD data looked at the occurrence of specific adverse events following more than 600,000 doses of HPV vaccination. The study found that females who received HPV vaccine were no more at risk of allergic reactions, anaphylaxis (severe allergic reaction), Guillain–Barré Syndrome (GBS), stroke, blood clots, appendicitis, or seizures than those who were unvaccinated or who received other vaccines. None of the adverse events found were any more common after HPV vaccination than among the comparison groups.
- A 2014 CDC report analyzed both prelicensure data and postlicensure safety data reported to VAERS following HPV vaccination from June 2006 through March 2014.
The prelicensure trails indicated that the proportions of persons who reported a serious adverse event, as well as the types of serious adverse events that were reported, were similar among both the vaccine and placebo groups. Additionally, reports of death were the same among the vaccinated group and the control group at less than 1% of participants, yet none, when investigated, were considered to be vaccine related.
The VAERS data indicated that 92.4% of reports were classified as non-serious. Among the 7.6% reports classified as serious, headache, nausea, vomiting, and fever were the most frequently reported symptoms. There were also 47 reports of confirmed death that were investigated. Causes of the confirmed death reports included bacterial meningitis, viral myocarditis, pulmonary embolism, diabetic ketoacidosis, and seizure disorder. A detailed review of every report of death identified no pattern with respect to time after vaccination, vaccine dose number, combination of vaccines administered, or diagnosis at death that would suggest a causal association with HPV vaccination.
- An Institute of Medicine (IOM) report reviewed published and unpublished studies on the safety of eight vaccines, including HPV and concluded that few health problems are caused by, or clearly associated with, vaccines. However, it did note that fainting may occur and that some people who have severe allergies to certain ingredients in vaccines could experience anaphylaxis, although this is very rare. An interview with Dr. Lauri Markowitz, of the Division of STD Prevention at the CDC, and published in Forbes suggested that people who are allergic to yeast should not get Gardasil 9 or Gardasil, and people who are allergic to latex should not get the bivalent vaccine Cervarix.
- A 2013 study that included almost 1 million girls found Gardasil was not associated with blood clots or adverse events related to the autoimmune and brain systems.
- A 2014 study that included over 1 million women found the vaccine was not associated with venous thromboembolism, also called VTE or blood clots.
- A 2012 study and a 2014 study both found women and girls who received the Gardasil shot were not more likely to develop autoimmune disorders than those who were unvaccinated.
- A recent JAMA study determined that there was no evidence to suggest that the HPV vaccine was associated with multiple sclerosis or the other demyelinating diseases.
The significance of these published studies, as well as numerous others, help illustrate how well researched HPV vaccines have been and the overwhelming evidence of their safety.
As a parent, if you’re frightened by a personal story of serious injury or even death following HPV vaccination, please consider the validity of the claims being made. Because it is in the public’s best interest to identify and expeditiously respond to any possible risks of the vaccine, there is a well established process for valid claims to be investigated here in the U.S. To date there has yet to be a death or major health concern validated by investigation.
9) HPV infections are declining as a direct result of vaccination.
It’s unacceptable that HPV vaccination rates are lagging behind in comparison to other vaccines recommended at the same age. Especially since there have been several studies that provide evidence that the vaccine is reducing the incidence of infection.
Consider a 2013 report by the CDC, which looked at some of the strains targeted by the vaccine. It compared the infection rate in girls 14-19 before the vaccine was approved, and another from after it was approved. Among girls who had received the vaccine, the drop in HPV infections was as high as 88%. However, when looking at all teens, both vaccinated and unvaccinated, the proportion of girls infected with the strains dropped by 56%, which suggests that as the population is properly vaccinated, there will be less spread of infection.
In a study of the Australian HPV vaccination program, where a high proportion of girls are vaccinated as recommended, there has been a sharp decline in the number of young women diagnosed with genital warts. By 2011, no genital warts were diagnosed in vaccinated women under 21 and less than 1% of all vaccinated and unvaccinated females under 21 years of age had genital warts in 2011, as compared to 10.5% of women in 2006 before the vaccination program started. This data, combined with a 44% reduction in the diagnosis of genital warts among young heterosexual men (who were not being vaccinated at the time), suggests that HPV infections are declining as a direct result of vaccination and the profound impact of herd immunity.
10) When parents skip or delay HPV vaccines, they’re contributing to the burden of cancer for the next generation.
If your doctor recommended a vaccine that could significantly reduce the risk of breast cancer in your daughter or prostate cancer for your son, would you refuse it? By getting your child the HPV vaccine series as recommended, you’re helping to prevent your child from suffering with cancer.
Years from now, when yet another generation is being diagnosed with HPV related cancers, how will we face our children? How will we accept the suffering, infertility and even death that we could have helped prevent?
For more information on HPV infection and vaccination, visit the following websites:
The Center for Disease Control and Prevention (CDC)